X chromosome inactivation serves as a fascinating biological mechanism that plays a critical role in balancing gene expression in females, who possess two X chromosomes. This intricate process is essential for regulating genes associated with X-linked diseases, such as Fragile X syndrome and Rett syndrome, both of which have significant implications in the realm of genetic disorders. Research spearheaded by Jeannie Lee has unveiled how the process of X chromosome inactivation occurs, providing hope for potential therapies that could alleviate the symptoms of these debilitating conditions. By uncovering the biochemical intricacies behind this chromosomal silencing, scientists are moving closer to developing innovative treatments that may one day help those affected by these and other X-linked diseases. As we delve deeper into the biology of X chromosome inactivation, we not only gain insights into fundamental genetic processes but also pave the way for groundbreaking advancements in the field of genetics and medicine.
The phenomenon known as X chromosome silencing, also referred to as lyonization, represents a pivotal aspect of gene regulation in women. This process ensures that one of the two X chromosomes in female cells is effectively turned off, preventing an overexpression of X-linked genes that could lead to various health issues. Researchers like Jeannie Lee have dedicated significant efforts to unraveling the complexities of this biological event, which significantly influences the understanding and treatment of genetic conditions such as fragile X and Rett syndromes. By investigating the mechanisms behind this unique form of chromosomal management, scientists hope to unlock new strategies for combating genetic disorders, providing fresh hope for effective therapies aimed at restoring gene functionality. Ultimately, exploring alternative terms like X-linked gene regulation sheds light on the broader implications of this vital genetic process.
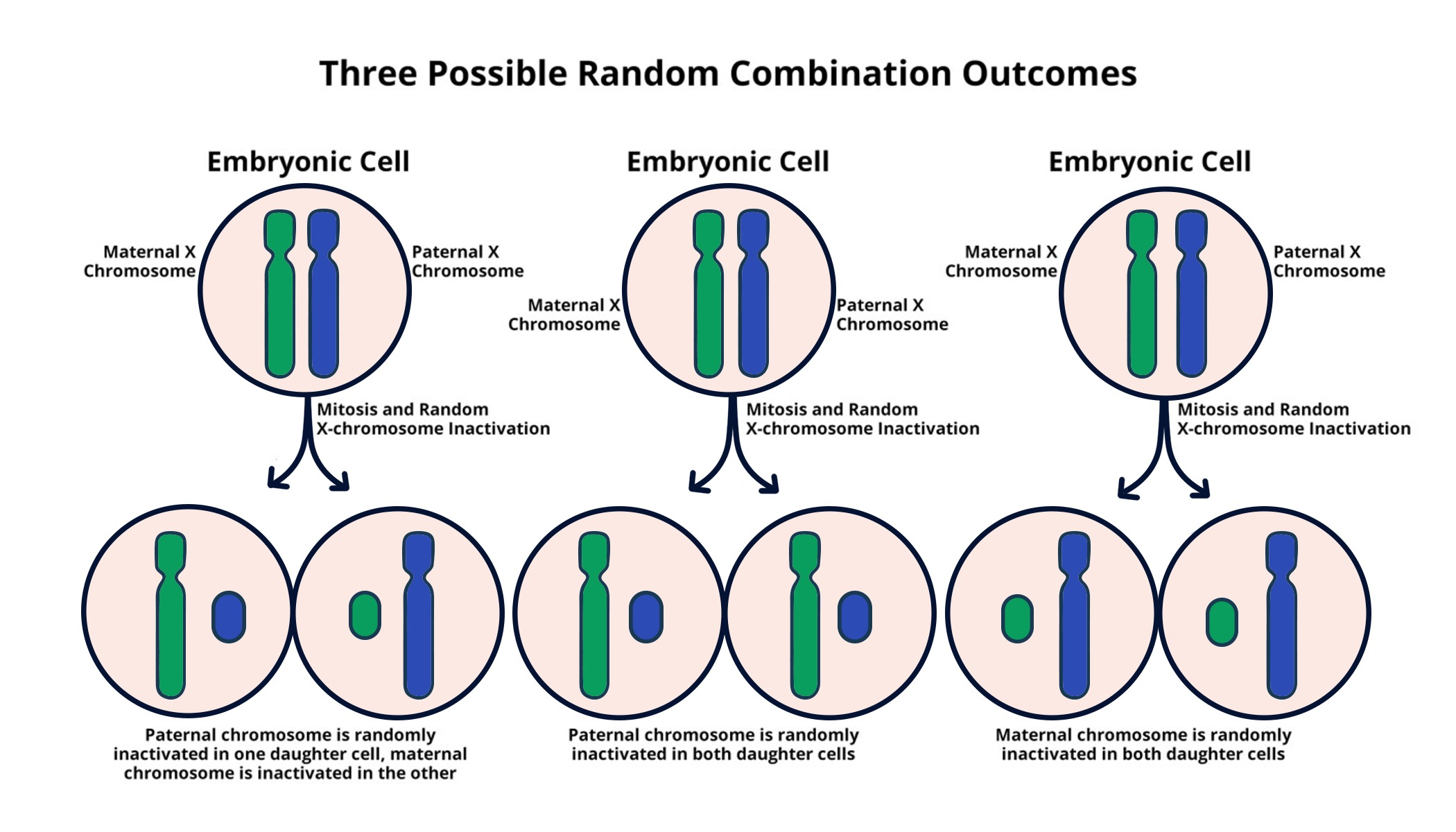
Understanding X Chromosome Inactivation: A Cellular Perspective
X chromosome inactivation (XCI) is a crucial biological process that ensures dosage compensation between males and females. In females, with two X chromosomes, one X is randomly silenced in early embryonic development, which allows for balanced gene expression within the cells. This genetic mechanism is not only fascinating from a fundamental research perspective, but it also holds significant implications in the context of various genetic disorders such as Fragile X syndrome and Rett syndrome. Jeannie Lee’s lab at Harvard is pioneering research in this area, unraveling the complexities of how this inactivation occurs and the potential therapeutic avenues it opens for treating X-linked diseases.
The recent findings from Lee’s lab shed light on the biophysical processes governing X chromosome inactivation. The research highlights the role of a gelatinous substance, likened to ‘Jell-O,’ that envelops the chromosome. This ‘Jell-O’ not only prevents chromosomes from tangling but is crucial for the binding and activity of Xist RNA, the molecule responsible for initiating the silencing of one X chromosome in females. Understanding these intricate details enhances nossa knowledge of cellular mechanisms and fuels the prospects for developing targeted gene therapies for disorders stemming from X-linked mutations.
Potential Therapies for Fragile X and Rett Syndromes
Fragile X syndrome and Rett syndrome are two prominent neurological disorders associated with mutations on the X chromosome. Current therapies aim at managing symptoms; however, recent advances in Jeannie Lee’s research suggest that it may be possible to reverse the underlying genetic issues fundamentally. By targeting the inactivated X chromosome and harnessing the potential of treatments that unsilence these genes, researchers can potentially restore normal gene function for individuals affected by these disorders. This approach signifies a shift from symptomatic treatment to addressing the source of the condition itself, thereby providing hope for better outcomes.
The therapeutic strategies being developed could also extend to males, who may carry mutations that lead to fragile X syndrome, despite not undergoing X chromosome inactivation. The emergence of methods to manipulate the Jell-O-like substance surrounding chromosomes could facilitate access to healthy gene copies, allowing restoration of function without impacting healthy genes. The goal is clear: to optimize these approaches and move towards clinical trials, ultimately providing solutions that could alter the trajectory of life for individuals affected by these X-linked diseases.
The Role of Jeannie Lee’s Research in Genetic Disorders
Jeannie Lee’s groundbreaking research serves as a cornerstone in our understanding of genetic disorders, particularly those tied to X chromosome abnormalities. As one delves deeper into her work, it becomes evident that the implications extend beyond just basic biology; they hover at the frontier of transformation in clinical practice. Her findings not only elucidate the mechanisms behind XCI but also pave the way for innovative treatments for conditions like Fragile X and Rett syndromes. As the research progresses toward clinical application, the potential for tangible therapeutic benefits becomes exceedingly optimistic.
Funded by the National Institutes of Health for over two decades, Lee’s lab has transitioned from exploring X chromosome inactivation mechanisms to understanding how these insights can help mitigate the impact of related genetic disorders. The transformation of basic research into therapeutic avenues illustrates the importance of sustained inquiry in genetics. By focusing on the X chromosome’s inactivation mechanism, Lee’s lab is positioned at the forefront of a new strategy that may significantly alter the treatment landscape for genetic disorders, embracing both the complexity of genes and the potential for innovative solutions.
The Gelatinous Barrier: Biophysics of X Chromosome Inactivation
The unique gelatinous substance that surrounds the X chromosome plays a fundamental role in the process of X chromosome inactivation (XCI). Its physical properties are crucial, as they determine how genes are expressed or silenced within the cell. As observed in Jeannie Lee’s research, this ‘Jell-O’ acts as a protective barrier that not only prevents entanglement of chromosomes but also creates an environment conducive to interactions with RNA molecules like Xist. Understanding this biophysical environment offers insights into why certain genes on the X chromosome can remain silenced while others can be activated.
Moreover, by manipulating the characteristics of this gelatinous coating, researchers can potentially influence the inactivation process, providing new avenues for gene therapy. If scientists can change the viscosity or other properties of this surrounding matrix, it may be possible to unmask critical genes that have been rendered inactive due to mutations, such as those involved in Fragile X and Rett syndromes. The research underscores the collaboration between biology and biophysics in understanding genetic mechanisms, highlighting the potential for interdisciplinary approaches to solving key medical challenges.
The Future of Genetic Disease Treatment: Insights from Jeannie Lee’s Work
As research into X chromosome inactivation continues to evolve, the future appears promising for the treatment of genetic diseases. The potential to lift the silencing of mutant genes on the X chromosome could revolutionize therapeutic strategies for conditions such as Fragile X syndrome and Rett syndrome. Jeannie Lee’s lab is not only uncovering the fundamental biology behind XCI but is also championing its translation into clinical applications, with ongoing efforts to optimize treatment protocols for clinical trials.
Furthermore, the implications of Lee’s findings extend beyond the traditional scope of female-centric genetic disorders. The strategies developed could assist male patients with X-linked disorders as well, prompting a broader re-evaluation of how we approach treatment for X-linked diseases. The core emphasis on genetic correction highlights a shift in medicine toward a more genetic and less symptomatic focus, promising to improve quality of life significantly for individuals suffering from these often debilitating conditions.
X-linked Diseases: Prioritizing Research Towards Solutions
The field of genetic research has increasingly recognized the significance of X-linked diseases, particularly given the unique challenges posed by X chromosome inactivation. Conditions like Fragile X syndrome and Rett syndrome, both highly prevalent, underscore the urgency for innovative research and therapeutic approaches. Jeannie Lee’s work serves as a critical reminder of the implications that understanding these genetic mechanisms can have on human health. By concentrating resources and knowledge on these areas, organizations can catalyze progress towards effective treatments.
By fostering a deeper understanding of X-linked diseases, researchers can engage in more targeted studies exploring potential pathways for new treatments. The findings emerging from Lee’s lab not only contribute to scientific knowledge but also serve as a beacon for future explorations into treatment options. Ultimately, prioritizing research in X-linked disorders promises to not only advance genetic science but also directly improve the lives of countless individuals affected by these conditions.
The Legacy of XCI Research in Modern Genetics
The legacy of X chromosome inactivation research is profound and far-reaching within the field of modern genetics. Jeannie Lee’s pioneering efforts have elucidated crucial insights into how gene expression is regulated differently between the sexes, laying the groundwork for understanding many X-linked disorders. This body of work highlights the complexities of genetic expression and reinforces the need for continued exploration in order to harness these insights for patient benefit.
As we stand on the cusp of potential breakthroughs, the discussions around XCI research continue to evolve, emphasizing the intersection of genetics, biophysics, and therapeutics. With an increased focus on developing interventions that can effectively reactivate or unsilence critical genes, the legacy of this research is not just about answering fundamental biological questions anymore; it is also about translating these answers into meaningful clinical impact for patients suffering from genetic disorders.
Toward Clinical Trials: The Path From Discovery to Treatment
The transition from discovery to treatment is a challenging journey in genetic research, particularly in the context of X-linked diseases. Jeannie Lee and her team are navigating this path with promising strategies aimed at unsilencing mutant genes associated with conditions like Fragile X and Rett syndromes. Their work is characterized by rigorous safety studies and optimization processes that are critical before any potential therapies can reach clinical trial stages.
This journey is not just about scientific exploration; it also involves ethical considerations, patient advocacy, and regulatory approval. As Lee’s lab prepares for the next steps, engaging with clinical stakeholders and the community will be vital. Highlighting the importance of transforming scientific findings into clinical realities emphasizes the collaborative effort necessary for successful therapeutic development, providing hope to families affected by X-linked genetic disorders.
Genetic Disorders and the Need for Continued Research
The landscape of genetic disorders is evolving, and with it, the necessity for continued research focused on understanding and treating these conditions. X-linked diseases, particularly, require a concerted effort to unravel their complexities and develop effective therapies. The research undertaken by Jeannie Lee’s lab highlights the importance of pursuing the basic science behind these disorders, as it builds a foundation for clinical advancements that could radically improve patient experiences.
By committing to thorough research into the mechanisms of X chromosome inactivation and exploring the therapeutic potential that arises, the scientific community can ensure that answers lead to actionable solutions. This commitment not only reflects a dedication to advancing genetic therapies but also emphasizes the potential to make lasting differences in many lives. Understanding genetic disorders through research allows for improved diagnostic methods and targeted interventions that can ultimately change the course of these diseases.
Frequently Asked Questions
What is X chromosome inactivation and how does it relate to Fragile X syndrome?
X chromosome inactivation is a biological process that occurs in female mammals, where one of the two X chromosomes is randomly silenced to prevent an overdose of X-linked gene products. This process is crucial for understanding genetic disorders like Fragile X syndrome, which is caused by a mutation on the X chromosome. The inactivation ensures that females with one mutated copy still have a functioning gene from the other X chromosome.
How does Jeannie Lee’s research on X chromosome inactivation impact the understanding of Rett syndrome?
Jeannie Lee’s research focuses on the mechanisms behind X chromosome inactivation, revealing how genes like Xist influence chromosomal silencing. Her findings have implications for Rett syndrome, a genetic disorder linked to mutations on the X chromosome, as they suggest potential therapies that could reactivate the silenced gene, restoring normal function.
What are the potential therapies for X-linked diseases arising from research on X chromosome inactivation?
Research on X chromosome inactivation has opened new avenues for potential therapies aimed at X-linked diseases, including Fragile X syndrome and Rett syndrome. Techniques being developed aim to unsilence inactive X chromosomes, thus making the healthy version of genes available for cellular use, offering hope for effective treatments.
Why is understanding X chromosome inactivation important for genetic disorders?
Understanding X chromosome inactivation is critical for tackling genetic disorders because it explains how mutations on one X chromosome can be masked or rendered inactive in females. By revealing how to manipulate this process, scientists can develop strategies to reactivate healthy genes, potentially providing cures for conditions like Fragile X syndrome and Rett syndrome.
What is the role of the Xist RNA molecule in X chromosome inactivation?
Xist is a critical RNA molecule that initiates X chromosome inactivation by modifying the surrounding chromosomal structure. It binds to the X chromosome and changes the physical properties of the chromatin, ultimately leading to the silencing of genes on that chromosome. This process is central to the study of X-linked diseases and potential therapeutic interventions.
Can therapies developed from X chromosome inactivation research be applied to males with X-linked diseases?
Yes, therapies developed from research on X chromosome inactivation can potentially benefit males as well. While males have only one X chromosome, certain gene mutations can still be silenced. Targeting the mechanisms of silencing could help reactivate healthy gene expression in males with X-linked diseases like Fragile X syndrome.
| Key Points |
|---|
| X chromosome presents challenges in females (two copies vs. males with one). |
| Females inactivate one X chromosome to balance gene dosage with males. |
| Jeannie Lee’s lab has uncovered the mechanism behind X chromosome inactivation. |
| Xist RNA molecule plays a crucial role in changing the Jell-O-like substance around the X chromosome to facilitate inactivation. |
| Understanding of X-inactivation could lead to potential therapies for Fragile X syndrome and Rett syndrome. |
| The goal is to unsilence inactivated X-linked genes to correct genetic disorders. |
| Research has been ongoing for two decades, with clinical applications emerging only recently. |
| No significant impact on genes not causing diseases during unsilencing, indicating potential for targeted therapy. |
Summary
X chromosome inactivation is a critical biological process that ensures females, having two X chromosomes, do not express double the dosage of X-linked genes compared to males. Recent research from Jeannie T. Lee’s lab reveals detailed mechanisms of this inactivation, highlighting the role of the Xist RNA and its interactions with chromosomal structures. This understanding opens up new possibilities for treating genetic disorders like Fragile X and Rett syndromes by potentially unsilencing healthy genes on the inactivated X chromosome. The implications of this work may provide therapeutic avenues that could benefit both females and males affected by these conditions.