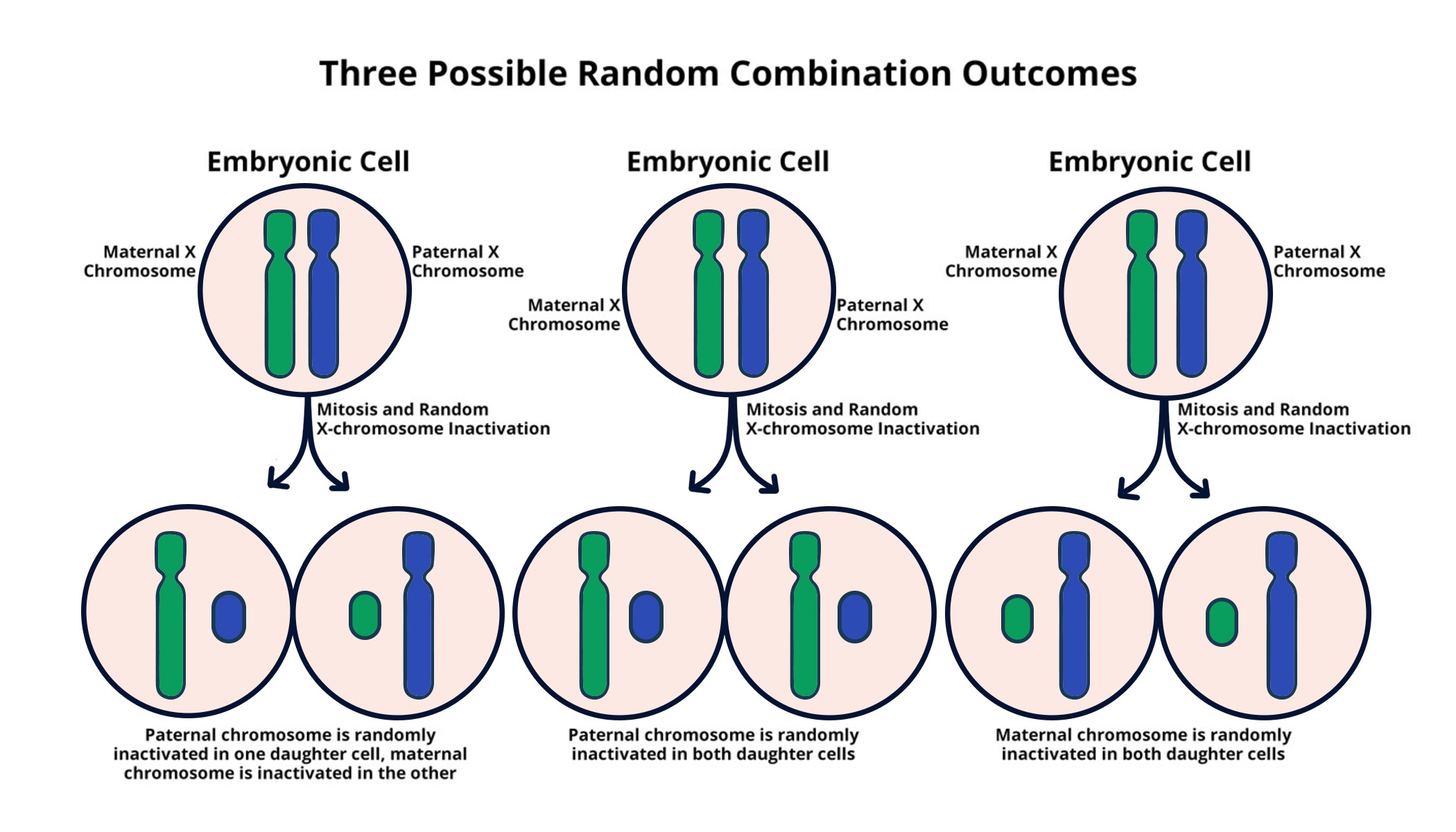
X chromosome inactivation (XCI) is a fascinating biological mechanism that ensures females, with their two X chromosomes, do not overexpress X-linked genes compared to males, who have only one. This unique process involves silencing one of the two X chromosomes in each cell, balancing the gene dosage crucial for normal development. Researchers like Jeannie T. Lee are unraveling the intricate details of XCI, especially its implications for chromosomal disorders such as Fragile X Syndrome and Rett Syndrome. Understanding X chromosome inactivation not only sheds light on fundamental genetics but also offers potential avenues for innovative genetic therapies aimed at treating these debilitating conditions. The ongoing studies may lead to reversing XCI, providing new hope for individuals affected by X-linked mutations and transforming the landscape of genetic medicine.
The inactivation of the X chromosome is a crucial video in understanding female genetic regulation, essentially ensuring that females do not express double the number of genes as males. This phenomenon of XCI acts as a regulatory mechanism, silencing one X chromosome to achieve a balance crucial for healthy development. Researchers, like Jeannie T. Lee, have been at the forefront of exploring this intriguing process, linking it to various chromosomal disorders including Fragile X and Rett syndromes. The exploration of X chromosome silencing opens up exciting possibilities for genetic therapies, potentially leading to innovative treatments for those impacted by these genetic disorders. As research progresses, understanding the nuances of XCI may pave the way for breakthroughs in reversing the effects of these debilitating conditions.
Understanding X Chromosome Inactivation: Key to Chromosomal Disorders
X chromosome inactivation (XCI) is a crucial biological process that occurs in female mammals, where one of the two X chromosomes is transcriptionally silenced. This mechanism is essential for maintaining a balance in gene dosage between males, who have only one X chromosome, and females. Jeannie T. Lee’s groundbreaking research has shed light on the complexities involved in this chromosomal silencing. Lee’s findings highlight how a gelatinous substance, akin to Jell-O, plays a pivotal role in the inactivation process, allowing cells to effectively manage their genetic resources. By understanding XCI, we can better grasp the underlying mechanisms of various chromosomal disorders, including Fragile X Syndrome and Rett Syndrome, which are often linked to mutations on the X chromosome.
The process of X-inactivation is not merely a random event; instead, it is a highly orchestrated sequence of molecular interactions. The production of the Xist RNA molecule triggers a series of transformations that facilitate the silencing of one X chromosome. Lee’s lab has made significant progress in elucidating how Xist interacts with the Jell-O-like substance surrounding chromosomes, effectively reshaping the physical environment of the X chromosome. This fundamental insight could pave the way for genetic therapies aimed at reversing the silence imposed on mutated genes, thus holding promise for treating debilitating conditions such as Fragile X Syndrome, characterized by emotional and intellectual challenges, and Rett Syndrome, which significantly affects girls’ development.
The Role of Jeannie T. Lee in Advancing Genetic Therapies
Jeannie T. Lee is at the forefront of research aiming to treat genetic disorders linked to X chromosome mutations. Her work not only addresses the fundamental mechanisms of X chromosome inactivation but also translates these insights into potential therapeutic interventions. By developing strategies to ‘unsilence’ X-linked genes, her lab offers hope for patients suffering from Fragile X Syndrome and Rett Syndrome. These conditions, which result from specific mutations on the X chromosome, lead to severe neurodevelopmental impairments and present a significant burden for families and healthcare systems alike.
The promise of Lee’s research extends beyond simply understanding X chromosome processes; it encompasses the innovative direction of genetic therapies that could effectively reverse the inactivation of mutated genes. As Lee states, her team is currently optimizing their methods and preparing for clinical trials, demonstrating a commitment to transitioning from laboratory research to potential real-world applications. This shift is critical as it brings the scientific community one step closer to developing actionable treatments that could alleviate symptoms and improve the quality of life for individuals with X-linked disorders.
Implications of Disrupting X Chromosome Inactivation
Disrupting X chromosome inactivation has profound implications, particularly for those with conditions like Fragile X Syndrome and Rett Syndrome. Understanding how to reactivate a single X chromosome could lead to exciting advances in treating these chromosomal disorders. The emerging therapies from Lee’s research indicate that it may be possible to selectively restore function to mutated genes while leaving healthy genes on the X chromosome unaffected. This specificity is crucial as it could lead to treatments with minimal side effects, a significant concern in current genetic therapy protocols.
Notably, the ability to reinstate the expression of mutated genes poses new questions about gene utilization in cells. Lee’s observations that cells may have a limited capacity to express genes hint at a sophisticated regulatory system within our DNA. This could explain why, when certain genes are reactivated, healthy gene expression remains stable even amid attempts to treat genetic disorders. As we further explore these implications, the focus on safe, efficient gene therapy can lead to new avenues in managing chromosomal disorders, fundamentally changing the landscape of genetic medicine.
Fragile X Syndrome: Causes and Treatment Options
Fragile X Syndrome (FXS) is one of the most common inherited causes of intellectual disability, resulting from a mutation in the FMR1 gene located on the X chromosome. This genetic disorder manifests itself through various developmental and psychological symptoms, making it a significant concern for affected individuals and their families. Research into the role of X chromosome inactivation by scientists like Jeannie T. Lee has underscored the necessity of understanding not just the causes but also the potential treatment avenues for FXS. Current approaches explore the disruption of XCI as a viable option to restore the function of the silenced FMR1 gene.
The development of genetic therapies aimed at FXS has become central to ongoing research efforts, with Lee’s lab leading initiatives to ‘unsilence’ this critical gene. The potential for using compounds to reverse the inactivation process could provide tangible relief for those affected by FXS. By focusing on the silencing mechanisms at play, researchers aim to develop targeted therapies that can effectively address this genetic disorder while minimizing adverse effects on other related genes. The promise of these therapies reaffirms the importance of continued investment in genetic research and underscores Lee’s vital role in advancing our understanding of and treatment options for Fragile X Syndrome.
Rett Syndrome: The Promise of Genetic Therapies
Rett Syndrome, another serious neurodevelopmental disorder predominantly affecting females, presents a unique challenge in the realm of genetic medicine. Caused by mutations in the MECP2 gene on the X chromosome, Rett Syndrome manifests through a decline in motor skills and cognitive abilities, creating a profound impact on quality of life. Recent findings related to X chromosome inactivation open new doors for exploring therapeutic options that may restore functionality to affected genes. Jeannie T. Lee’s research illustrates how strategies developed in her lab could serve as pathways to innovative treatments.
The potential of genetic therapies for Rett Syndrome resides in their ability to ‘unsilence’ the inactivated MECP2 gene, offering hope for restoring the lost functions that define this debilitating disorder. By advancing the understanding of how Xist RNA interacts with chromosomal structures, researchers are laying the groundwork for therapies that could transform patient outcomes. As these therapeutic approaches move toward clinical trials, they represent a hopeful frontier in the search for effective treatments for Rett Syndrome, highlighting the importance of interdisciplinary research in genetics and therapeutic development.
Challenges in Genetic Research and Therapy Development
The journey of exploring X chromosome inactivation and its implications for genetic disorders is not without its challenges. While substantial progress has been made in understanding the mechanisms governing this process, translating laboratory discoveries into effective therapies presents a significant hurdle. Jeannie T. Lee’s studies emphasize that despite promising initial findings, the complex nature of gene regulation and interactions within the chromosomal environment mean that strategies must be meticulously refined before advancing to clinical applications. The research community must navigate the nuances of gene therapies, ensuring they are safe and effective for a diverse range of patients.
Moreover, understanding how to selectively target the right genes while preserving normal function is a critical challenge. The potential for reactivating genes linked to conditions like Fragile X and Rett Syndrome raises questions not only about efficacy but also about long-term impacts. Continuing investment in rigorous clinical trials and safety studies will be crucial to overcoming these obstacles. Lee advocates for a systematic approach, underpinning the necessity of thorough research to achieve breakthroughs in the ambitious endeavor to develop targeted genetic therapies for chromosomal disorders.
Future Directions in Genetic Research
Looking ahead, the future of genetic research, particularly concerning X chromosome inactivation, is poised for exciting advancements. Researchers like Jeannie T. Lee set the stage for groundbreaking therapies that have the potential to transform the treatment landscape for genetic disorders associated with X chromosome mutations. As methods to unsilence X-linked genes become more refined, the implications for conditions like Fragile X Syndrome and Rett Syndrome could lead to effective interventions that address root causes rather than just symptoms.
Furthermore, the ongoing exploration of the role of chromosomal structure, such as the gelatinous substance that facilitates X inactivation, offers rich avenues for innovation in genetic therapies. The insights gained through this research may extend beyond X-linked disorders, influencing broader strategies in treating other genetic conditions. As we continue to unravel the complexities of chromosomal behavior, the much-anticipated breakthroughs in the coming years could define new paradigms in genetic medicine, providing hope to millions affected by hereditary disorders.
The Importance of Funding in Genetic Research
The expansion of genetic research and the development of novel therapeutic strategies greatly depend on sustained funding from institutions and government bodies. Jeannie T. Lee’s research, which has received support from the National Institutes of Health, serves as a testament to the critical role that financial backing plays in advancing complicated scientific inquiries that require long-term investment. Without adequate funding, groundbreaking studies that have the potential to revolutionize treatments for conditions like Fragile X Syndrome and Rett Syndrome might not reach their full potential or even come to fruition.
Moreover, increased financial support can enhance collaboration among researchers across various fields, encouraging a multi-disciplinary approach to developing comprehensive solutions for genetic disorders. Funding also enables the construction of better facilities, more extensive equipment, and a larger research team, facilitating innovative experiments and accelerating the pace of discovery. As we witness the ongoing advancements in genetic research, it is evident that robust funding frameworks are essential to catalyzing change and ensuring that insights lead to tangible therapeutic options for those in need.
Frequently Asked Questions
What is X chromosome inactivation and its significance in genetic disorders?
X chromosome inactivation (XCI) is a biological process where one of the two X chromosomes in female mammals is randomly inactivated during early embryonic development. This process ensures that females, despite having two X chromosomes, express genes from only one. Its significance in genetic disorders lies in its association with conditions such as Fragile X Syndrome and Rett Syndrome, where mutations on the X chromosome can lead to various developmental and cognitive challenges.
How does Jeannie T. Lee’s research contribute to understanding X chromosome inactivation?
Jeannie T. Lee’s research has significantly advanced our understanding of X chromosome inactivation by elucidating the mechanisms involved in this complex process. Her lab discovered how the RNA molecule Xist plays a crucial role in altering the properties of the surrounding chromosomal material, ultimately leading to the silencing of one X chromosome. This fundamental insight provides a potential pathway for developing genetic therapies for conditions like Fragile X Syndrome and Rett Syndrome.
What are the potential therapeutic implications of reversing X chromosome inactivation for Fragile X and Rett syndromes?
Reversing X chromosome inactivation could have groundbreaking therapeutic implications for conditions such as Fragile X Syndrome and Rett Syndrome. By unsilencing the inactive X chromosome, it may become possible to restore the function of healthy genes that were previously inaccessible due to their inactivation. This approach holds promise for treating genetic disorders linked to mutations on the X chromosome, potentially alleviating the symptoms associated with these conditions.
What role does the ‘Jell-O-like’ substance play in X chromosome inactivation?
The ‘Jell-O-like’ substance surrounding the chromosomes is essential for X chromosome inactivation. In Jeannie T. Lee’s research, this gelatinous material is shown to create a flexible environment that allows the RNA molecule Xist to penetrate and modify it, leading to the silencing of one of the X chromosomes in female cells. This mechanism helps prevent the chromosomes from entangling and supports the orderly inactivation process.
How might genetic therapies based on X chromosome inactivation benefit males with X-linked mutations?
Genetic therapies based on X chromosome inactivation could benefit males with X-linked mutations, such as those causing Fragile X Syndrome, by employing similar mechanisms to silence mutated genes. By potentially reactivating the healthy version of a gene on the X chromosome, therapies could help both males and females by providing access to functional genes lost due to mutations, leading to improved cellular function and health outcomes.
| Key Points | Details |
|---|---|
| X Chromosome Inactivation | Females have two X chromosomes, but only one is active due to inactivation. |
| Role of Xist | A gene on the X chromosome produces an RNA molecule called Xist, which is critical in the inactivation process. |
| Gelatinous Substance | The ‘Jell-O-like’ substance surrounds chromosomes, preventing them from tangling and facilitating X chromosome silencing. |
| Potential Treatments | Research aims to reverse X-inactivation, offering potential cures for Fragile X Syndrome and Rett Syndrome. |
| Applications Beyond Females | Similar mechanisms may help address X-linked mutations in males. |
| Current Research | Ongoing optimization and safety tests of therapies to be transitioned into clinical trials. |
Summary
X chromosome inactivation is a crucial biological process that allows females to function with just one active X chromosome. The intricate mechanisms of this process, as explored by Jeannie Lee’s research, reveal the potential for significant therapeutic advancements, particularly in genetic disorders such as Fragile X and Rett syndromes. By unraveling how Xist and surrounding substances interact to silence one of the X chromosomes, researchers are paving the way toward innovative treatments that could benefit both females and males affected by X-linked conditions.